Medical Genome Reference Bank – Data Access
Curated data will be openly accessible to the international research community.
To maintain participant privacy and confidentiality, whilst maximising MGRB utility, we have deployed a tiered data management system that determines the richness of data that is made available to researchers. This consists of three access tiers; Open access, Controlled access and Restricted access, summarised in the schematic below.
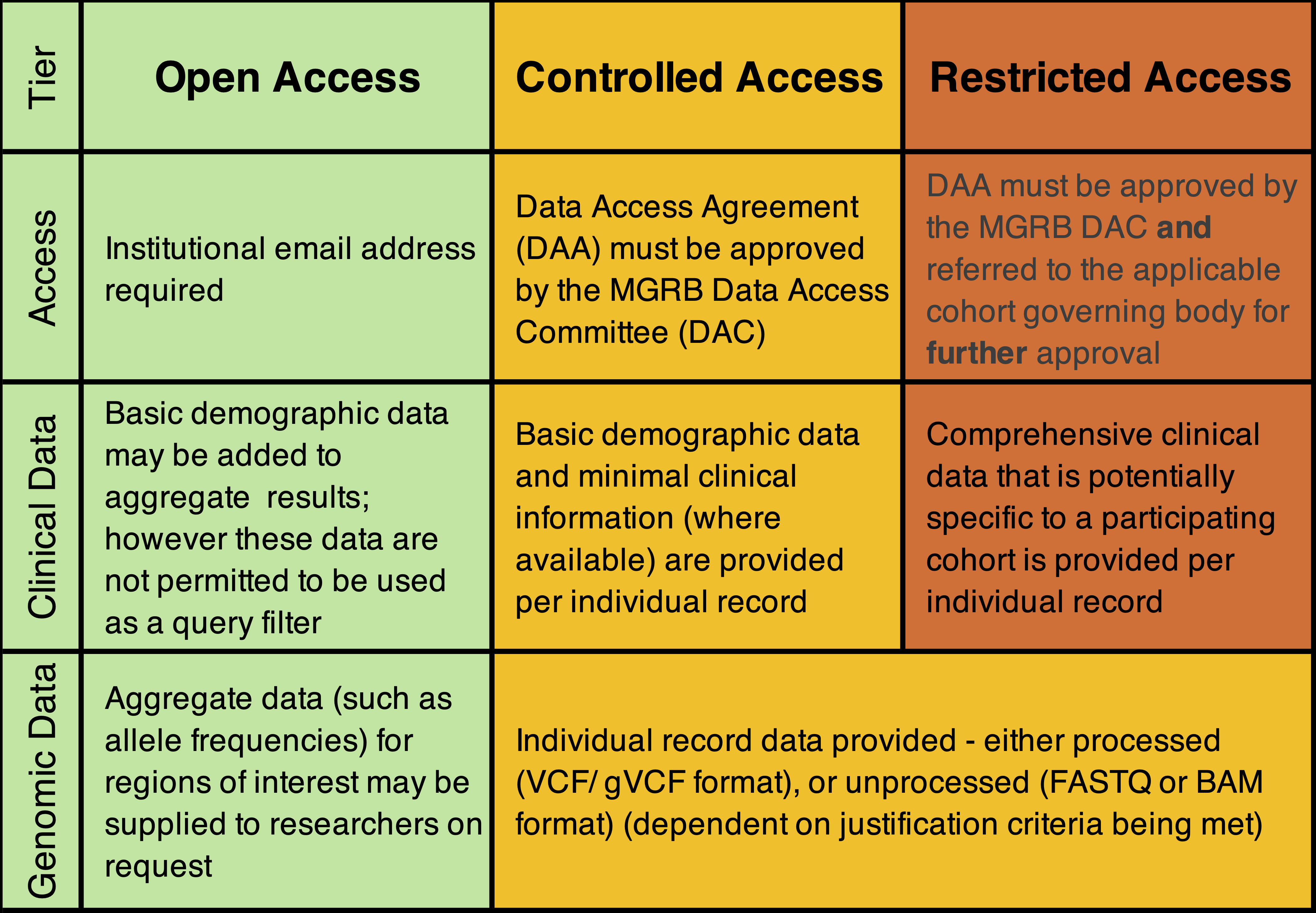
There are three documents that govern the Medical Genome Reference Bank data access:
- The Data Access Policy (DAP) summarises the governance policy applied to individual and institutional access to the Medical Genome Reference Bank (MGRB).
- The Information Handling Statement (IHS) describes how private information is handled in the MGRB project.
- The Data Access Application (DAA) is an application to gain access to comprehensive genotypic and clinical information from MGRB participants. This application is based on the exacting standards of the European Genome-phenome Archive.
Open access requests: please send an email message with details of the request to mgrb@garvan.org.au. There is no requirement to use the DAA form.
Controlled or Restricted access: please use the DAA form.The completed form must be submitted through the Garvan REMS system. A step-by-step guide is here.
 SYDNEY GENOMICS COLLABORATIVE
SYDNEY GENOMICS COLLABORATIVE